Striking effect of low-dose radiotherapy combined with PD-1 blockade on small cell lung cancer in mice and refractory patients (Achilles Study)
Background: Immune checkpoint inhibitors (ICIs) provide a durable and long-term benefit but still unsatisfactory clinical efficacy for extensive-stage small cell lung cancer (ES-SCLC). An optimized dose and schedule of radiotherapy for ES-SCLC remain to be studied. We hypothesized that the addition of low-dose radiotherapy (LDRT) to ICI could improve the efficacy of ES-SCLC. Methods: In SCLC bearing mice, the tumor was irradiated with LDRT in dose-escalation starting at 3Gy×1 fractions (f) up to 3Gy×7f, and combined with PD-1 antibody injection (0.2mg/mouse, i.p, every 3 days). Mice were followed for tumor growth and survival. The tumor microenvironment was dynamically analyzed every 3 days till day 18 by flow cytometry and immunofluorescence. 15 patients (pts) with relapsed ES-SCLC who received the treatment with LDRT and PD-1 blockade were retrospectively reviewed. Results: LDRT with 3Gy×5f delivered over 5 days (LDRT15Gy/5f) was determined to be the optimized dose when combined with PD-1 blockade. Combined group exhibited obvious growth retardation and prolonged survival compared to either monotherapy.The most robust infiltration of T cells in combined group was observed on day 9 after start of treatment. Bulk and single-cell RNA-seq showed significant immune cells infiltration, predominately CD8+ T cells and M1 macrophage. The activation and degranulation of CD8+ T cells in combination group were enhanced. Intra-tumoral CD8+ T cells were mainly assigned to effector memory (Tem) cells, then exhausted precursor (Texp) cells and exhausted (Tex) cells. Pseudo-time analysis supported a state evolution model that CD8+ Tem cells differentiate into intra-tumoral CD8+ Tex cells through an intermediate CD8+ Texp state. Tumor cells were divided into clusters 0, 1, and 2. Cluster 0 nearly disappeared while the proportion of cluster 1 increased in combination therapy. Cluster 1 enriched inflammatory response pathways and higher expression of MHC class I versus clusters 0 and 2. Additionally, the density of intra-tumoral microvessel was decreased, while vascular perfusion and the number of pericyte-covered vessels increased in combined group. Correspondingly, 15 recurrent ES-SCLC pts who treated with up-front LDRT15Gy/5f plus PD-1 blockade showed an ORR of 80%. Median PFS and OS were 4.3 months and 10.9 months. PFS rates at 6, 12 and 24 months were 40%, 20% and 6.7%, and OS rates were 60%, 40% and 24%, respectively. No patient experienced above grade 4 radiation- and immunotherapy-related toxicity. Conclusions: Our study is the first report of the synergistic effect of LDRT15Gy/5f and PD-1 blockade in SCLC mice model and recurrent ES-SCLC pts. The LDRT15Gy/5f demonstrates both immunologic adjuvant and cytotoxic effect on SCLC, and is safe and feasible in clinical pts. Further translational research, Match trial (NCT04622228), is ongoing.
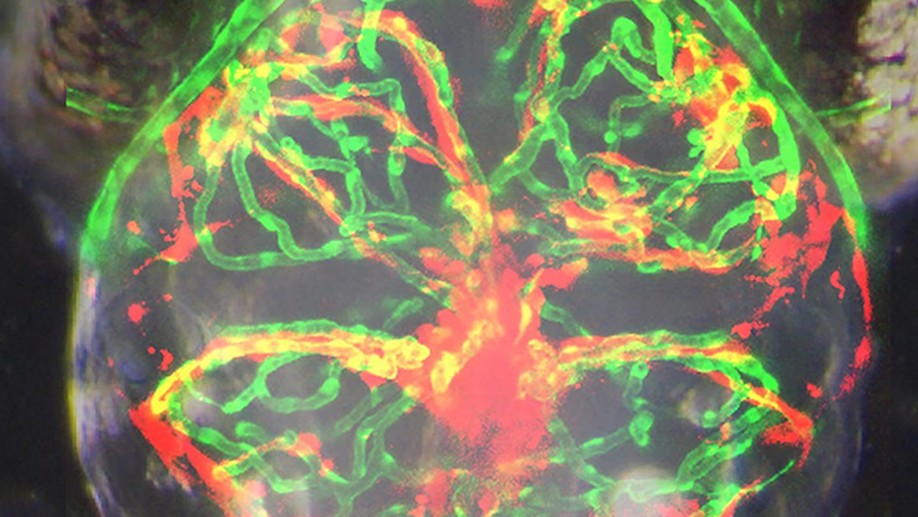
Clinically relevant orthotopic xenograft models of patient-derived glioblastoma in zebrafish
An accurate prediction of the intracranial infiltration tendency and drug response of individual glioblastoma (GBM) cells is essential for personalized prognosis and treatment for this disease. However, the clinical utility of mouse patient-derived orthotopic xenograft (PDOX) models remains limited given current technical constraints, including difficulty in generating sufficient sample numbers from small tissue samples and a long latency period for results. To overcome these issues, we established zebrafish GBM xenografts of diverse origin, which can tolerate intracranial engraftment and maintain their unique histological features. Subsequent single-cell RNA-sequencing (scRNA-seq) analysis confirmed significant transcriptional identity to that of invading GBM microtumors observed in the proportionally larger brains of model animals and humans. Endothelial scRNA-seq confirmed that the zebrafish blood-brain barrier is homologous to the mammalian blood-brain barrier. Finally, we established a rapid and efficient zebrafish PDOX (zPDOX) model, which can predict long-term outcomes of GBM patients within 20 days. The zPDOX model provides a novel avenue for precision medicine of GBM, especially for the evaluation of intracranial infiltration tendency and prediction of individual drug sensitivity.
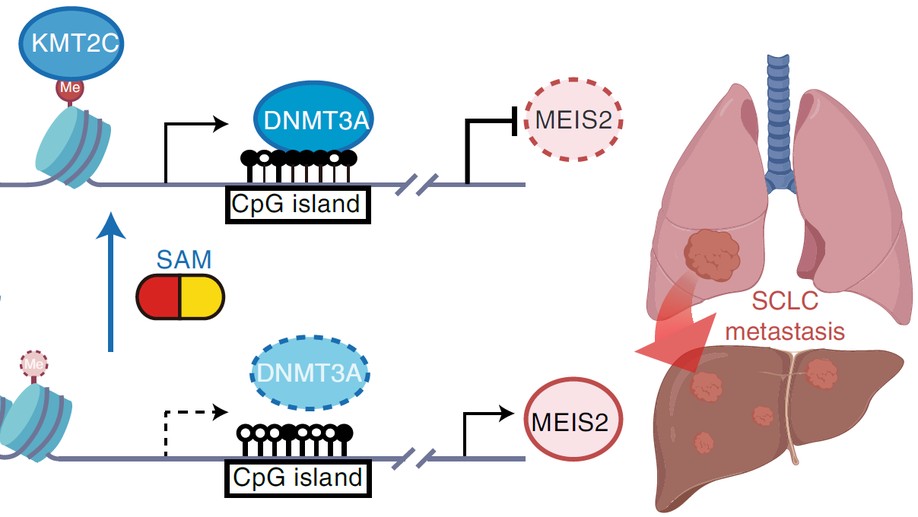
KMT2C deficiency promotes small cell lung cancer metastasis through DNMT3A-mediated epigenetic reprogramming
Small cell lung cancer (SCLC) is notorious for its early and frequent metastases, which contribute to it as a recalcitrant malignancy. To understand the molecular mechanisms underlying SCLC metastasis, we generated SCLC mouse models with orthotopically transplanted genome-edited lung organoids and performed multiomics analyses. We found that a deficiency of KMT2C, a histone H3 lysine 4 methyltransferase frequently mutated in extensive-stage SCLC, promoted multiple-organ metastases in mice. Metastatic and KMT2C-deficient SCLC displayed both histone and DNA hypomethylation. Mechanistically, KMT2C directly regulated the expression of DNMT3A, a de novo DNA methyltransferase, through histone methylation. Forced DNMT3A expression restrained metastasis of KMT2C-deficient SCLC through repressing metastasis-promoting MEIS/HOX genes. Further, S-(5′-adenosyl)-l-methionine, the common cofactor of histone and DNA methyltransferases, inhibited SCLC metastasis. Thus, our study revealed a concerted epigenetic reprogramming of KMT2C- and DNMT3A-mediated histone and DNA hypomethylation underlying SCLC metastasis, which suggested a potential epigenetic therapeutic vulnerability.
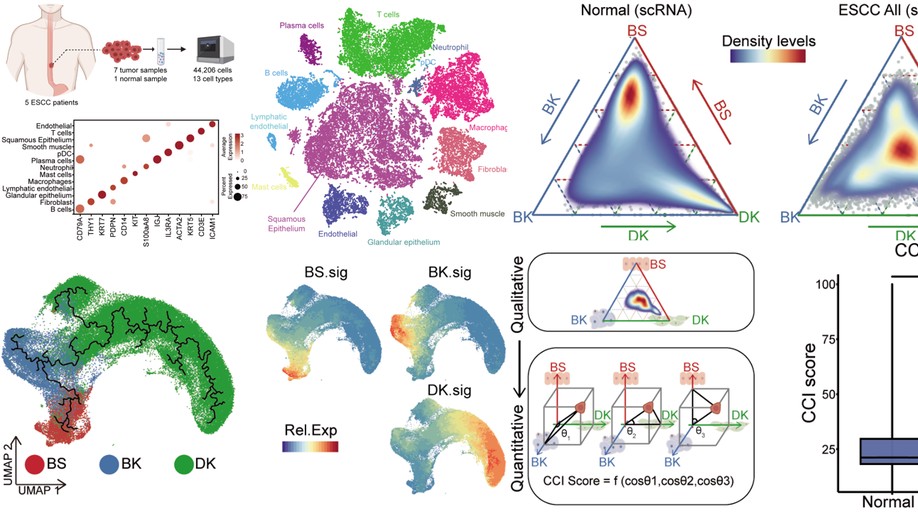
Identifying a confused cell identity for esophageal squamous cell carcinoma
The cell identity of malignant cells and how they acquire it are fundamental for our understanding of cancer. Here, we report that esophageal squamous cell carcinoma (ESCC) cells display molecular features equally similar but distinct to all three types of normal esophageal epithelial cells, which we term as confused cell identity (CCI). CCI is an independent prognostic marker associated with poor prognosis in ESCC. Further, we identify tropomyosin 4 (TPM4) as a critical CCI gene that promotes the aggressiveness of ESCC in vitro and in vivo. And TPM4 creates CCI through activating the Jak/STAT-SOX2 pathway. Thus, our study suggests an unrecognized feature of ESCC cells, which might be of value for clinic prognosis and potential interference.
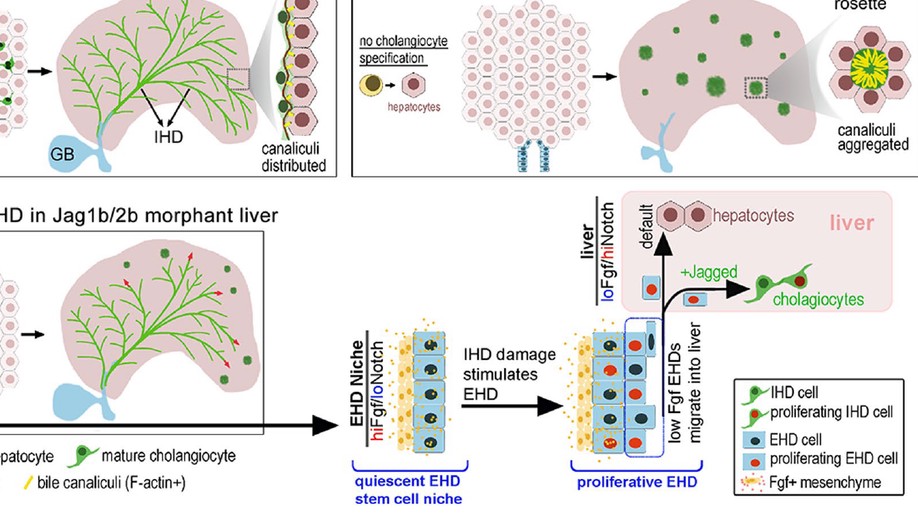
Intrahepatic cholangiocyte regeneration from an Fgf-dependent extrahepatic progenitor niche in a zebrafish model of Alagille Syndrome
Background and aims: Alagille Syndrome (ALGS) is a congenital disorder caused by mutations in the Notch ligand gene JAGGED1, leading to neonatal loss of intrahepatic duct (IHD) cells and cholestasis. Cholestasis can resolve in certain patients with ALGS, suggesting regeneration of IHD cells. However, the mechanisms driving IHD cell regeneration following Jagged loss remains unclear. Here, we show that cholestasis due to developmental loss of IHD cells can be consistently phenocopied in zebrafish with compound jagged1b and jagged2b mutations or knockdown. Approach and results: Leveraging the transience of jagged knockdown in juvenile zebrafish, we find that resumption of Jagged expression leads to robust regeneration of IHD cells through a Notch-dependent mechanism. Combining multiple lineage tracing strategies with whole-liver three-dimensional imaging, we demonstrate that the extrahepatic duct (EHD) is the primary source of multipotent progenitors that contribute to the regeneration, but not to the development, of IHD cells. Hepatocyte-to-IHD cell transdifferentiation is possible but rarely detected. Progenitors in the EHD proliferate and migrate into the liver with Notch signaling loss and differentiate into IHD cells if Notch signaling increases. Tissue-specific mosaic analysis with an inducible dominant-negative Fgf receptor suggests that Fgf signaling from the surrounding mesenchymal cells maintains this extrahepatic niche by directly preventing premature differentiation and allocation of EHD progenitors to the liver. Indeed, transcriptional profiling and functional analysis of adult mouse EHD organoids uncover their distinct differentiation and proliferative potential relative to IHD organoids. Conclusions: Our data show that IHD cells regenerate upon resumption of Jagged/Notch signaling, from multipotent progenitors originating from an Fgf-dependent extrahepatic stem cell niche. We posit that if Jagged/Notch signaling is augmented, through normal stochastic variation, gene therapy, or a Notch agonist, regeneration of IHD cells in patients with ALGS may be enhanced.
Intrahepatic cholangiocyte regeneration from an Fgf-dependent extrahepatic progenitor niche in a zebrafish model of Alagille Syndrome
Coronavirus disease 2019 (COVID-19) has brought about a great threat to global public health. Recently, a new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant B.1.1.529 has been reported in South Africa and induced a rapid increase in COVID-19 cases. On November 24, 2021, B.1.1.529 named Omicron was designated as a variant under monitoring (VUM) by World Health Organization (WHO). Two days later, the Omicron variant was classified as a variant of concern (VOC). This variant harbors a high number of mutations, including 15 mutations in the receptor-binding domain (RBD) of spike. The Omicron variant also shares several mutations with the previous VOC Alpha, Beta, and Gamma variants, which immediately raised global concerns about viral transmissibility, pathogenicity, and immune evasion. Here we described the discovery and characteristics of the Omicron variant, compared the mutations of the spike in the five VOCs, and further raised possible strategies to prevent and overcome the prevalence of the Omicron variant.
An Epigenetic Mechanism Underlying Chromosome 17p Deletion-Driven Tumorigenesis
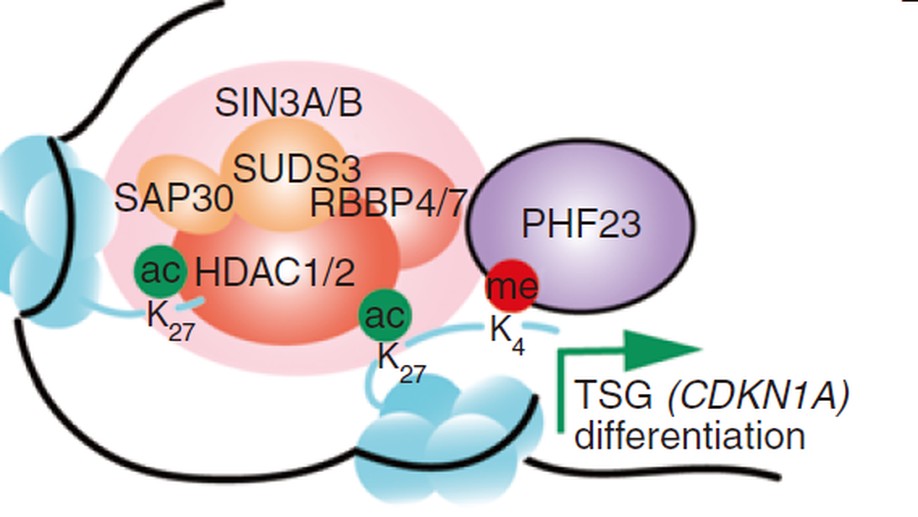
Chromosome copy-number variations are a hallmark of cancer. Among them, the prevalent chromosome 17p deletions are associated with poor prognosis and can promote tumorigenesis more than TP53 loss. Here, we use multiple functional genetic strategies and identify a new 17p tumor suppressor gene (TSG), plant homeodomain finger protein 23 (PHF23). Its deficiency impairs B-cell differentiation and promotes immature B-lymphoblastic malignancy. Mechanistically, we demonstrate that PHF23, an H3K4me3 reader, directly binds the SIN3-HDAC complex through its N-terminus and represses its deacetylation activity on H3K27ac. Thus, the PHF23-SIN3-HDAC (PSH) complex coordinates these two major active histone markers for the activation of downstream TSGs and differentiation-related genes. Furthermore, dysregulation of the PSH complex is essential for the development and maintenance of PHF23-deficient and 17p-deleted tumors. Hence, our study reveals a novel epigenetic regulatory mechanism that contributes to the pathology of 17p-deleted cancers and suggests a susceptibility in this disease.
Nivolumab treatment of relapsed/refractory Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis in adults
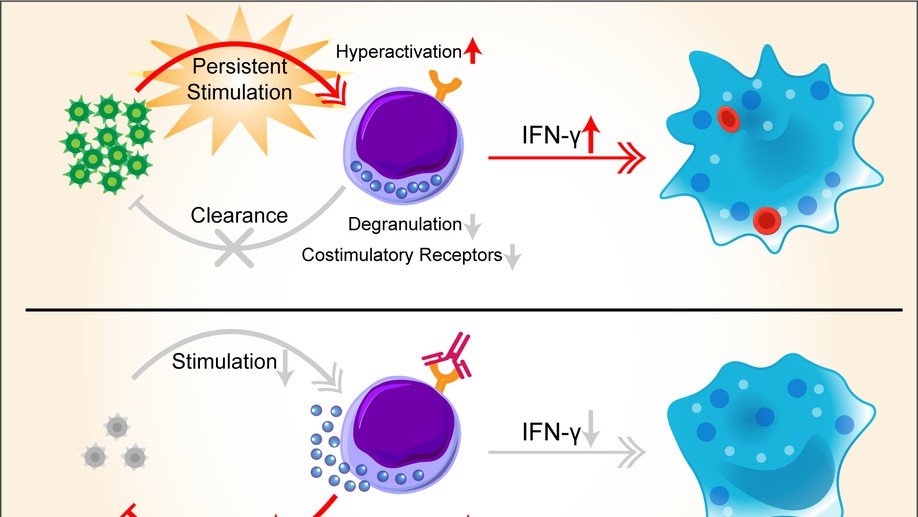
Epstein-Barr virus (EBV)-associated hemophagocytic lymphohistiocytosis (EBV-HLH) is a life-threatening hyperinflammatory syndrome triggered by EBV infection. It often becomes relapsed or refractory (r/r), given that etoposide-based regimens cannot effectively clear the virus. r/r EBV-HLH is invariably lethal in adults without allogeneic hematopoietic stem cell transplantation. Here, we performed a retrospective analysis of 7 r/r EBV-HLH patients who were treated with nivolumab on a compassionate-use basis at West China Hospital. All 7 patients tolerated the treatment and 6 responded to it. Five of them achieved and remained in clinical complete remission with a median follow-up of 16 months (range, 11.4-18.9 months). Importantly, both plasma and cellular EBV-DNAs were completely eradicated in 4 patients. Single-cell RNA-sequencing analysis showed that HLH syndrome was associated with hyperactive monocytes/macrophages and ineffective CD8 T cells with a defective activation program. Nivolumab treatment expanded programmed death protein-1–positive T cells and restored the expression of HLH-associated degranulation and costimulatory genes in CD8 T cells. Our data suggest that nivolumab, as a monotherapy, provides a potential cure for r/r EBV-HLH, most likely by restoring a defective anti-EBV response.
Epigenetic drug library screening identified an LSD1 inhibitor to target UTX-deficient cells for differentiation therapy
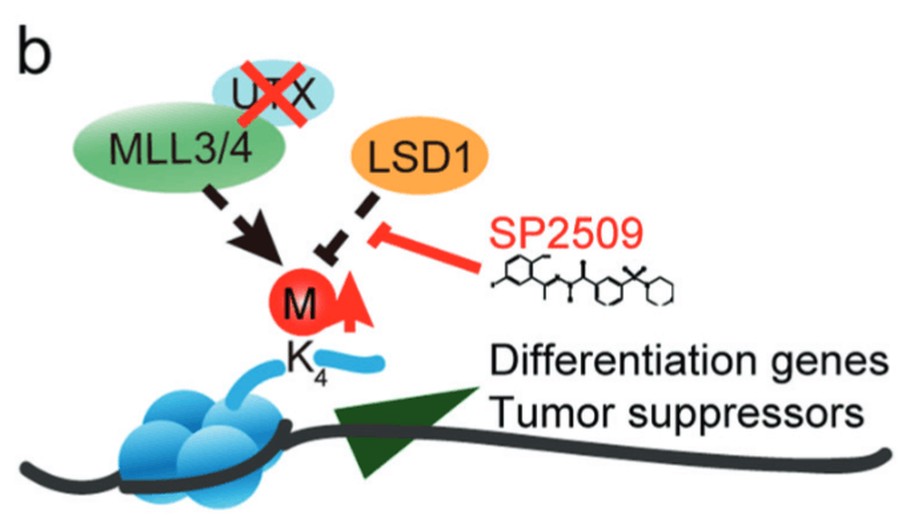
UTX (also known as KDM6A), a histone 3 lysine 27 demethylase, is among the most frequently mutated epigenetic regulators in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Recent studies have suggested that UTX mutations promote MDS and AML by blocking the differentiation of hematopoietic stem and progenitor cells (HSPCs). Here, we performed an epigenetic drug library screening for small molecules able to release the differentiation block on HSPCs induced by UTX deficiency. We found that SP2509, a selective inhibitor of LSD1, specifically promoted the differentiation of Utx-null HSPCs while sparing wild-type HSPCs. Transcriptome profiling showed that Utx loss reduced the expression of differentiation-related and tumor suppressor genes, correlating with their potential roles in HSPC self-renewal and leukemogenesis. In contrast, SP2509 treatment reversed these changes in gene expression in Utx-null HSPCs. Accordingly, Utx loss decreased H3K4 methylation level probably through the COMPASS-like complex, while LSD1 inhibition by SP2509 partially reversed the reduction of H3K4 methylation in Utx-deficient HSPCs. Further, SP2509 promoted the differentiation of Utx-null AML cells in vitro and in vivo and, therefore, extended the survival of these leukemic mice. Thus, our study identified a novel strategy to specifically target both premalignant and malignant cells with Utx deficiency for differentiation therapy and provided insights into the molecular mechanisms underlying the role of Utx in regulating HSPCs and related diseases.